Incidental Finding of Raised CA125: a Cause for Concern?
Farshad Tahmasebi*, Rahul Nath, Nava Sokolovsky, Johannah Scaffidi, Jane Boley, Gautam Mehra and Ahmad Sayanseh
DOI10.21767/2471-9803.1000170
1Department of Gynaecological Oncology, St Thomas’ Hospital, London, UK
2School of Life Course Sciences, King’s College London, Guy’s, Kings College and St. Thomas’ Hospital, London, UK, School of Medical Education, King’s College, London, UK
- *Corresponding Author:
- Farshad Tahmasebi
Department of Gynaecological Oncology, St Thomas’ Hospital
London, UK
E-mail: tfarshad@hotmail.com
Received date: October 30, 2018; Accepted date: November 15, 2018; Published date: November 21, 2018
Citation: Tahmasebi F, Nath R, Sokolovsky N, Scaffidi J, Boley J, et al. (2018) Incidental Finding of Raised CA125: a Cause for Concern? Crit Care Obst Gyne Vol.5 No.1:3. doi: 10.21767/2471-9803.1000170
Copyright: © 2018 Tahmasebi F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cancer antigen 125 (also known as carbohydrate antigen 125 or CA 125) is an antigen first identified by Bast et al.
Although much research has been done to understand the molecular structure of CA 125, its function remains a source of much speculation. At present, CA 125 is most commonly used in the diagnosis of epithelial non-mucinous ovarian cancers, and in monitoring their response to treatment. Following surgical resection, CA 125 levels are expected to half within 10 days and it is therefore a useful tool for assessing response to treatment as well.
We present a case of incidental finding of rasied CA125, followed by the literature review to discuss a better understanding of the factors that lead to CA 125 production, its mechanisms of action, and the nature and functions of its subspecies may help in improving its use as a standalone diagnostic tool.
Keywords
CA 125; Ovarian cancer; Pancreatic cancer
Introduction
Cancer antigen 125 (also known as carbohydrate antigen 125 or CA 125) is an antigen belonging to a family of high-molecularweight O-glycosylated proteins known as mucins. It is found on cell surfaces and is the largest transmembrane mucin. It was first identified by Bast, et al. who isolated the murine monoclonal antibody OC125 that detects CA 125, so named because it was the 125th antibody produced against the ovarian cancer cell line. Since then, numerous other monoclonal antibodies have been found that target CA125, though OC125 remains the best known [1]. CA 125 is encoded by the MUC16 gene located on chromosome 19 and is therefore also known as MUC16 [2]. Normal levels of CA 125 are considered to be <35 U/ml with most commonly used assays [3].
Although much research has been done to understand the molecular structure of CA 125, its functions remain a source of much speculation. It is expressed in tissues derived from embryonic coelomic epithelium such as endometrium, Mullerian epithelium, peritoneum, pleura and pericardium [4]. Within these epithelia, it is synthesized by mesothelial cells in response to various stimuli, most notably mechanical stress and inflammation [5,6]. It has been posited that CA 125 may play a role in cell-mediated immune response (e.g., by suppressing the response of Natural Killer cells) and in promoting attachment to mesothelial cells by binding to mesothelin [7,8]. Interestingly, several subspecies of CA 125 have been described, but it is yet unclear whether these different subspecies are associated with different physical conditions [8].
At present, CA 125 is most commonly used in the diagnosis of epithelial non-mucinous ovarian cancers, and in monitoring their response to treatment. It is elevated in most cases of epithelial ovarian cancer, and particularly in those involving peritoneal deposits, and is therefore used as an adjunct to Transvaginal Ultrasonography (TVUSS) in the diagnosis of ovarian tumors. However, its levels are normal in 50% of women with stage I disease and in most women with occult cancers identified at prophylactic surgery [9]. It is also not usually elevated in cases of non-epithelial ovarian cancers. Following surgical resection, CA 125 levels are expected to half within 10 days and it is, therefore, a useful tool for assessing response to treatment as well [10]. Nevertheless, CA 125 has also repeatedly been shown to be elevated in a multitude of other malignant and nonmalignant conditions, especially when there is an element of serosal fluid involvement. Therefore, although it has relatively good sensitivity, CA 125 lacks specificity and as such, is not useful as a screening tool or a standalone measurement [11].
A case of falsely elevated CA 125
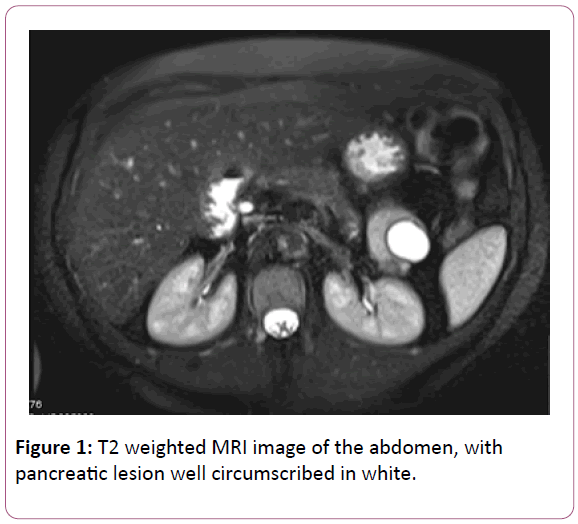
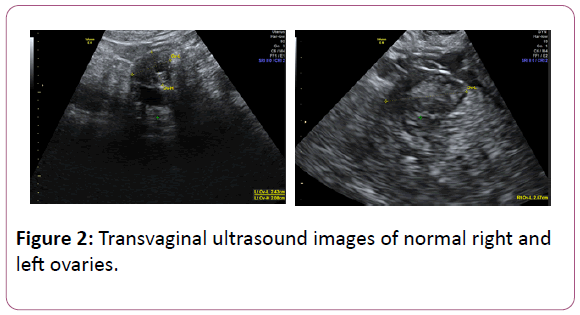
We recently encountered the case of a 55-year-old premenopausal woman, who was referred to our Rapid Access Gynae-Oncology Clinic with a raised CA 125 on two separate occasions, measured three months apart (79 kU/l and 55 kU/L). Her past medical history was significant only for a cholecystectomy and two previous vaginal deliveries. TVUSS was unremarkable. An MRI revealed an enlarged multi fibroid uterus with features suggestive of adenomyosis, as well as a 2.4 × 2.8 cm multiloculated T2 hyperintense lesion within the tail of the pancreas. The ovaries appeared normal.
Further investigation of the pancreatic mass with pancreatic MRI and endoscopic ultrasound suggested a well-circumscribed mixed solid/cystic lesion arising from the tail of the pancreas, measuring 4.5 × 3.7 cm (Figure 1). CA 199 was found to be elevated (79 U/ml; normal range 0-27 kU/L). The note was also made of two indeterminate sub-pleural lung nodules with a maximal diameter of 6 mm. It was not possible to perform fine needle aspiration of the pancreatic lesion due to the splenic artery obstructing the access field. Following consultation with an upper gastrointestinal specialist, the decision was made to remove the lesion and the patient underwent partial pancreatectomy and splenectomy. The lesion was found to be benign on histology.
Despite the above intervention, her CA 125 levels continued to be elevated with a fluctuating pattern, ranging from 78 kU/L to 620 kU/L. Repeated TVUSS scans were unchanged with ongoing evidence of multiple fibroids and adenomyosis only (Figure 2). Consistent with this, she noted a history of menorrhagia and was found to have iron-deficiency anemia. No further intervention was undertaken at this time.
Eighteen months after her surgery, she was admitted to hospital with an acutely ischaemic left lower limb secondary to occlusion of dorsalis pedis and posterior tibial artery. Extensive investigations showed multiple small nodules in the lung bases and fatty infiltration of the liver, but with no concerning cause for the arterial thromboembolism. She was treated with anticoagulation for a total of 8 months before switching to longterm aspirin. Further follow-up in the gynecology clinic showed on-going elevated levels of CA 125 (241 kU/L, 339 kU/L, 177 kU/L) and stable ultrasonographic appearances.
Raised CA 125 without ovarian cancer
Numerous studies have demonstrated alternative causes for raised CA 125 levels, both physiological and pathological. Johnson, et al. used data from a large cancer screening trial in the United States to assess lifestyle factors that can increase CA 125 in a cohort of postmenopausal women [12]. They found higher levels in women with a history of smoking, previous breast cancer, and use of hormone replacement therapy, although in most women these levels were still within the normal range. Multiple studies have shown a more significant rise in CA 125 levels in pregnancy [13,14]. Levels have also been repeatedly demonstrated to fluctuate across the menstrual cycle, with the highest levels seen during menstruation followed by a progressive decline until the end of the cycle [15-17]. This fluctuation is not seen in women who have undergone hysterectomy with ovarian preservation [18], suggesting that these cyclic changes are related to the endometrium. Indeed, in vitro studies have shown that CA 125 is produced in higher concentrations in endometrial stromal cells during the proliferative and early secretory phases, indicating that production of CA 125 by these cells is associated with estrogendominated cell growth and activity [19]. One theory is that, during menstruation, the tissue-blood barrier is temporarily weakened and therefore CA 125 from endometrial cells is released into the bloodstream with a corresponding rise in its serum levels [17,18]. Nevertheless, despite these cyclical changes, CA 125 levels remain mostly within the normal range in the vast majority of women.
Serum CA 125 levels have however been shown to rise significantly above the normal range in several malignant conditions. Elevated levels of the marker have been found in malignancies of cervix, uterus, breast, lung and many gastrointestinal tract organs including liver and pancreas [11]. Expression of CA 125 has also been shown to be high in vitro in samples of pancreatic, oesophageal, colorectal and gastric cancer tissues, but is absent in benign tissues from these same organs [20].
Similarly, CA 125 has been consistently shown to be elevated in a variety of benign conditions. These include gynecological pathologies such as endometriosis, pelvic inflammatory disease [14], and ovarian hyperstimulation syndrome [21]. Several nongynecological conditions have also been shown to be associated with significantly raised CA 125 levels, including liver cirrhosis [22-24]; lung diseases such as tuberculosis and interstitial lung disease [25]; and heart diseases such as heart failure, atrial fibrillation and pericardial disease [6,26,27]. Interestingly, in patients with heart disease, CA 125 appears to be a good candidate for a risk stratification marker as particularly high levels have been shown to be associated with a high risk of rehospitalization and death [6]. In both the benign and malignant conditions mentioned above, the actual level of CA 125 can reach into the hundreds (U/ml). However, one possible differentiating factor is that levels in malignancy tend to progressively rise with time, whereas in benign conditions they display a more stable pattern, which may fluctuate with disease severity [11].
Production of CA 125 in response to stress
To better understand why CA 125 is raised in such a diverse range of conditions, it is crucial to first understand the mechanisms that stimulate its production. The most popular current theories suggest that CA 125 is synthesized by mesothelial cells in response to stress, which can be either mechanical stress caused by fluid overload, or inflammatory stress instigated by the release of mediators such as TNFα and interleukins [5,6].
Numerous studies have demonstrated that CA 125 elevation is closely linked to the presence of fluid overload in serosal spaces, regardless of its etiology. Huang, et al. looked at patients with pulmonary edema, with or without evidence of associated Chronic Heart Failure (CHF) [26]. They found that CA 125 levels were higher in all patients with pleural effusion, regardless of whether this was secondary to CHF or not. Interestingly, they also found a moderate correlation between raised CA125 levels and decreasing albumin, which is also associated with fluid overload. Similar findings have been shown in patients with ascites, where CA 125 levels were elevated both in association with benign conditions such as liver cirrhosis [23] and malignancies such as digestive tract tumors [24].
In a study that included a more varied selection of conditions, Topalak, et al. [22] assessed patients with both gynecological and non-gynecological diseases, including hepatic disease, lung cancer, and non-gynecological peritoneal carcinomatosis. Patients were divided into those with and those without features of peritoneal or pleural effusion. They again noted that raised CA 125 was seen in multiple conditions other than ovarian cancer, and this was particularly the case when effusions were present. In these latter cases, CA 125 levels could reach >300 U/ml. However, the highest levels were still seen in patients with ovarian cancer and resultant ascites, where there was also a positive correlation between the amount of ascites and CA 125 level. These findings suggest that CA 125 is not of tumor origin, but is instead released by mesothelial cells as a response to the mechanical stretch caused by fluid accumulation. It is therefore not a tumor marker per se. Indeed, measurements of CA 125 in the ascetic fluid have been shown to be correlated with, but much higher than, levels in serum, suggesting that it originates in ascetic fluid rather than in cancerous tissue, from whence it spreads into the bloodstream [24]. In vitro studies have demonstrated that mechanical stretch of mesothelial cells causes upregulation of the MUC16 gene, lending further support to this theory [26].
Other evidence suggests that CA 125 may also be released in response to inflammatory stress, which would explain its elevation in cases where there is no evidence of serosal effusion [6]. For example, in patients with heart failure, CA 125 levels can be elevated even when the condition is well controlled and there is no evidence of pulmonary or peripheral edema [7]. High levels have also been found in a case of subhepatic abscess [28], again highlighting the possible role of inflammation in stimulating CA 125 release. Indeed, in vitro studies have demonstrated secretion of CA 125 in response to stimulation with TNFα and interleukins [7].
Cases of falsely elevated CA 125
Given its lack of specificity, the value of an isolated finding of elevated CA 125 levels without additional evidence of abnormal pathology remains dubious. In fact, several cases have been described of abnormally high values with no clinical abnormality, despite thorough assessment.
Watt, et al. investigated the case of a 41-year-old woman with a persistently raised CA 125 of greater than 300 U/ml (using the Abbott MEIA assay) [29]. However, when levels were tested with a different assay (IRMA), they were within the normal range. In vitro studies suggested that the false positive results were due to the interaction of the animal sera used in the MEIA assay with the patient’s own antibodies, which may have cross-reacted with the assay as the patient was a veterinarian and therefore frequently exposed to animals. Interestingly though, she underwent a total hysterectomy and bilateral salpingooophorectomy for a finding of a simple right ovarian cyst, and although histology confirmed benign appearances, CA 125 returned to normal levels with the MEIA assay post-operativelya finding the authors were unable to explain. Nevertheless, it has also been shown that the presence of anti-mouse antibodies in a patient’s serum can cause false positive results in some assays [30].
Hosono, et al. investigated five women with significantly raised CA 125 levels, ranging from 193 to 5461 U/ml, who were all clinically well with unremarkable scans [31]. They found that the antigenic nature of the CA 125 present in these women’s sera differed from that found in women with ovarian cancer as it lacked the CA 130 epitope. CA 130 is an epitope found on the same antigen molecule as CA 125, and the two are usually closely correlated. The possibility, therefore, arises that there are different antigen molecules containing CA 125 which may be associated with different functions and therefore diseases.
CA 125 in pancreatic cancer
In the case of our patient described above, the pancreatic mass that was removed was found to be benign on histology and it may well be argued that the surgical intervention was unnecessary or excessive. However, evidence exists of an association between raised CA 125 levels and pancreatic cancer, although it is rarely used as a biomarker for this type of malignancy in practice due to its lack of sensitivity and specificity [32]. Studies looking at the expression of MUC16 in pancreatic tissue samples show that it is upregulated in the majority of ductal adenocarcinomas and high-grade pre-malignant dysplasias (pancreatic intraepithelial neoplasias), but absent in healthy pancreatic cells [20,33]. Its expression also increases progressively with advancing cancer grade. Moreover, there is an association between more diffuse MUC16 expression in tissue and worse patient outcome, defined as shorter survival time following diagnosis [20].
The difficulty with these in vitro studies is that they look at the expression of CA 125 in tissues, and it is not clear whether these levels would be reflected in serum measurements. Studies looking at its expression in serum indicate that high levels are seen in only 45-63% of patients with pancreatic cancer [20,34]. Nevertheless, pancreatic cancer is notorious for its poor survival rates with a 5-year survival of only 6% [35], and therefore one could argue that the combination of raised CA 125 levels and a pancreatic mass could be deemed suspicious enough to justify surgical intervention.
CA 125 in endometriosis
Of possible relevance to our case report, with a history of menorrhagia and radiological evidence of adenomyosis, numerous studies have demonstrated a link between raised CA 125 levels and endometriosis [36-38]. CA 125 levels are significantly higher in patients with endometriosis stage II or above when compared with healthy controls, and can reach into the hundreds of units/ml [19]. Levels have also been shown to increase with disease severity and may be predictive of significant pelvic adhesions in these patients [37]. Furthermore, despite being raised above normal limits, CA 125 levels in patients with endometriosis show similar changes across the menstrual cycle to healthy controls [38].
Conclusion
Overall, it is clear from the evidence detailed here that isolated elevated levels of CA 125 with no other clinical or radiological evidence of significant pathology are neither sensitive nor specific enough to warrant immediate concern or justify invasive treatment. In our case, the fluctuating CA 125 levels are more suggestive of a benign pathology or may possibly be insignificant altogether. Possible further investigations in such a case may be testing for different antigenic determinants (e.g., CA130) to identify whether the abnormally high levels are secondary to a CA 125 molecule of different antigenic nature. Another option would be to monitor CA 125 levels in relation to the menstrual cycle, as it is possible that the raised levels are secondary to adenomyosis and/or endometriosis, and typical cyclical variation would support this hypothesis. However, such investigations would be more academic than clinical and we argue that the best option would be to minimize invasive procedures unless a clear indication arises, and instead adopt a “watchful wait” approach with minimally invasive monitoring.
In conclusion, we suggest that CA 125 should not be performed as an isolated test with no clear indication as it is neither specific nor sensitive enough to lead to a diagnosis on its own. It should not, therefore, be used as a screening tool, as appears to have been the case in our patient. When used in this manner, it can result in unnecessary investigations and invasive treatments and can lead to significant anxiety for the patient. A better understanding of the factors that lead to CA 125 production, its mechanisms of action, and the nature and functions of its subspecies may help in improving its use as a standalone diagnostic tool, but at present, our knowledge of this molecule remains insufficient for this purpose.
Author Contributions
AS and FT were involved in the conception of this case report and literature search following the care of the involved patient and prompting the paper to be created. AS and FT were involved in data collection for the case report, which was written by JS. FT and NS performed the literature review. AS, FT, JS, and NS edited the case report and literature review and wrote the introduction, as well as creating the initial draft of the article. JB and RN were involved in writing the manuscript and finalizing the paper. Final approval of the article was a joint contribution between all listed authors.
Acknowledgments
None
Conflicts of Interest
None.
References
- Bast RC Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, et al. (1981) Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest 68: 1331-1337.
- Yin BW, Lloyd KO (2001) Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. J Biol Chem 276: 27371-27375.
- Shih M, Sokoll L, Chan DW (2002) Ovarian cancer. In: Diamandis EP, Fritsche HA, Lilja H, Chan DW, Schwartz MK (eds.) Tumor Markers: Physiology, pathobiology, technology and clinical applications. Washington DC: AACC Press, pp: 239-252.
- Kabawat SE, Bast RC Jr, Bhan AK, Welch WR, Knapp RC, et al. (1983) Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC 125. Int J Gynecol Pathol 2: 275-285.
- Bischof P (1993) What do we know about the origin of CA 125. Eur J Obstet Gynecol Reprod Biol 49: 93-98.
- Falcao F, de Oliveira FRA, da Silva MCFC, Sobral Filho DC (2018) Carbohydrate antigen 125: a promising tool for risk stratification in heart diseases. Biomark Med 12: 367-381.
- Núñez J, Miñana G, Núñez E, Chorro FJ, Bodí V, et al. (2014) Clinical utility of antigen carbohydrate 125 in heart failure. Heart Fail Rev 19: 575-584.
- Scholler N, Urban N (2007) CA125 in ovarian cancer. Biomark Med 1: 513-523.
- Fotopoulou C, Sehouli J, Ewald-Riegler N, de Gregorio N, Reuss A, et al. (2015) The value of serum CA125 in the diagnosis of borderline tumors of the ovary: A subanalysis of the prospective multicenter ROBOT study. Int J of Gynec Canc 25: 1248-1252.
- Bidart JM, Thuillier F, Augereau C, Chalas J, Daver A, et al. (1999) Kinetics of serum tumor marker concentrations and usefulness in clinical monitoring. Clin Chem 45: 1695-1707.
- Reiter MJ, Costello JE, Schwope RB, Lisanti CJ, Osswald MB (2015) Review of commonly used serum tumor markers and their relevance for image interpretation. J Comput Assist Tomogr 39: 825-834.
- Johnson CC, Kessel B, Riley TL, Ragard LR, Williams CR, et al. (2008) The epidemiology of CA-125 in women without evidence of ovarian cancer in the Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) screening trial. Gynec Onc 110: 383-389.
- Niloff JM, Knapp RC, Schaetzl E, Reynolds C, Bast RC Jr. (1984) CA125 antigen levels in obstetric and gynecologic patients. Obstet Gynecol 64: 703-707.
- Halila H, Stenman UH, Seppala M (1986) Ovarian cancer antigen CA 125 levels in pelvic inflammatory disease and pregnancy. Cancer 57: 1327-1329.
- McLemore MR, Aouizerat BE, Lee KA, Chen LM, Cooper B, et al. (2012) A comparison of the cyclic variation in serum levels of CA125 across the menstrual cycle using two commercial assays. Biol Res Nursing 14: 250-256.
- Bon GG, Kenemans P, Dekker JJ, Hompes PG, Varstraeten RA, et al. (1999) Fluctuations in CA 125 and CA 15-3 serum concentrations during spontaneous ovulatory cycles. Hum Repr 14: 566-570.
- Falconer H, Bambra CS, Chai D, Cornillie FJ, Hill JA, et al. (2005) The effect of endometriosis, cycle stage, lymphocyte suppression and pregnancy on CA-125 levels in peritoneal fluid and serum in baboons. Hum Reprod 20: 3033-3038.
- Kafali H, Artunc H, Erdem M (2007) Evaluation of factors that may be responsible for cyclic change of CA125 levels during menstrual cycle. Arch Gynecol Obstet 275: 175-177.
- Bischof P, Tseng L, Brioschi PA, Herrmann WL (1986) Cancer antigen 125 is produced by human endometrial stromal cells. Hum Repr 1: 423-426.
- Streppel MM, Vincent A, Mukherjee R, Campbell NR, Chen SH, et al. (2012) Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol 43: 1755-1763.
- Jager W, Diedrich K, Wildt L (1987) Elevated levels of CA-125 in serum of patients suffering from ovarian hyperstimulation syndrome. Fertil Steril 48: 675-678.
- Topalak O, Saygili U, Soyturk M, Karaca N, Batur Y, et al. (2002) Serum, pleural effusion and ascites CA-125 levels in ovarian cancer and nonovarian benign and malignant diseases: a comparative study. Gynec Onc 85: 108-113.
- Qureshi MO, Dar FS, Khokhar N (2014) Cancer antigen-125 as a marker of ascites in patients with liver cirrhosis. J Coll Physicians Surg Pak 24: 232-235.
- Xiao WB, Liu YL (2003) Elevation of serum and ascites cancer antigen 125 levels in patients with liver cirrhosis. J Gastroent and Hepatol 18: 1315-1316.
- Wang T, Zheng XJ, Ji YL, Liang ZA, Liang BM (2016) Tumour markers in rheumatoid arthritis-associated interstitial lung disease. Clin Exp Rheumatol 34: 587-591.
- Huang F, Zhang K, Chen J, Cai Q, Liu X, et al. (2013) Elevation of carbohydrate antigen 125 in chronic heart failure may be caused by mechanical extension of mesothelial cells from serous cavity effusion. Clin Biochem 46: 1694-1700.
- Durak-Nalbantic A, Resic N, Kulic M, Pecar E, Zvizdic F, et al. (2013) Serum level of tumor marker carbohydrate antigen-CA125 in heart failure. Med Arch 67: 241-244.
- Kasalický P, Kocián J (1998) CA 125 in the department of internal medicine-a marker of ovarian tumor, thoracic fluid or ascites? Vnitr Lek 44: 478-480.
- Watt PD, Rosen B, Bunting P, D’Costa M, Malkin A, et al. (1994) Consequences of a falsely elevated CA 125. Clin Biochem 27: 419-420.
- Oei AL, Sweep FC, Massuger LF, Olthaar AJ, Thomas CM (2008) Transient human anti-mouse antibodies (HAMA) interference in CA 125 measurements during monitoring of ovarian cancer patients treated with murine monoclonal antibody. Gynaecol Oncol 109: 199-202.
- Hosono MN, Endo K, Sakahara H, Watanabe Y, Saga T, et al. (1992) Different antigenic nature in apparently healthy women with high serum CA125 levels compared with typical patients with ovarian cancer. Cancer 70: 2851-2856.
- Brody JR, Witkiewicz AK, Yeo CJ (2011) The past, present, and future of biomarkers: A need for molecular beacons for the clinical management of pancreatic cancer. Advances in Surgery 45: 301-321.
- Haridas D, Chakraborty S, Ponnusamy MP, Lakshmanan I, Rachagani S, et al. (2011) Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS One 6: e26839.
- Haglund C (1986) Tumour marker antigen CA125 in pancreatic cancer: a comparison with CA 19-9 and CEA. Br J Cancer 54: 897-901.
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics. CA Cancer J Clin 60: 277-300.
- Colacurci N, Fortunato N, De Franciscis P, Fratta M, Cioffi M, et al. (1996) Serum and peritoneal CA-125 levels as diagnostic test for endometriosis. Eur J Obstet Gynecol Reprod Biol 66: 41-43.
- Cheng YM, Wang ST, Chou CY (2002) Serum CA-125 in preoperative patients at high risk for endometriosis. Obstet Gynecol 99: 375-380.
- Hornstein MD, Thomas PP, Gleason RE, Barbieri RL (1992) Menstrual cyclicity of CA-125 in patients with endometriosis. Fertil Steril 58: 279-283.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences