Gestational Weight Gain in a Woman with Peripartum Cardiomyopathy
Ayako Sakamoto, Takeshi Umazume, Mamoru Morikawa, Satoshi Ishikawa, Takahiro Yamada1, Satoshi Yamada and Hisanori Minakami
DOI10.21767/2471-9803.100028
Ayako Sakamoto1, Takeshi Umazume1, Mamoru Morikawa1, Satoshi Ishikawa1, Takahiro Yamada1, Satoshi Yamada2 and Hisanori Minakami1*
1Department of Obstetrics, Hokkaido University Graduate School of Medicine, Sapporo, Japan
2Department of Cardiovascular Medicine, Hokkaido University Graduate School of Medicine, Sapporo, Japan
- *Corresponding Author:
- Hisanori Minakami
Department of Obstetrics
Hokkaido University Graduate School of Medicine
N14 W6, Kita-ku
Sapporo, Japan
Tel: +0353876547398
E-mail: minasho@med.hokudai.ac.jp
Received date: June 19, 2016; Accepted date: July 6, 2016; Published date: July 13, 2016
Citation: Sakamoto A, Umazume T, Morikawa M, et al. Gestational weight gain in a woman with peripartum cardiomyopathy. Crit Care Obst&Gyne. 2016, 2:4. doi: 10.2176 7/2471-9803.100028
Copyright: © 2016 Sakamoto A et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
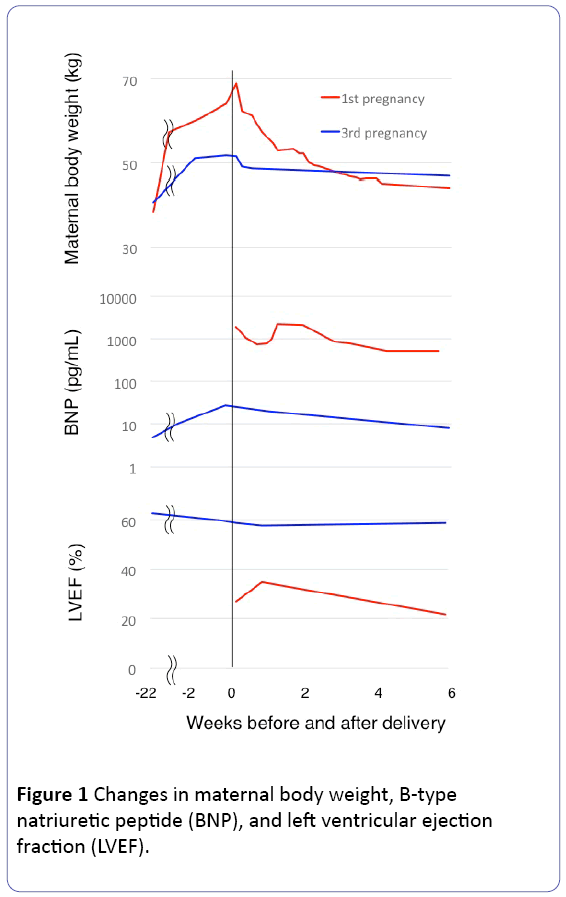
Greater gestational weight gain (GWG) may be a risk factor for peripartum cardiomyopathy (PPCM). In counseling, women with a prior history of PPCM associated with greater GWG should be advised to avoid excessive GWG in subsequent pregnancies. The third pregnancy occurred in a 36-year-old Japanese woman with height of 1.48 m and left ventricular ejection fraction (LVEF) of 62% following the first and second pregnancies complicated with PPCM and spontaneous abortion, respectively. She exhibited GWG of 14.7 kg (from 37.0 to 51.7 kg) and normal or nearly normal plasma B-type natriuretic peptide levels and LVEF (>55%) throughout the current pregnancy. In her first pregnancy at the age of 33 years, extraordinary GWG of 30 kg (from 38.5 to 69.1 kg) preceded PPCM with nadir LVEF of 22% at 6 weeks postpartum followed by more than 18-month LVEF of <55%.
Keywords
Biomarker; Cardiomyopathy; Weight gain, Echocardiography; Pregnancy complication
Introduction
Peripartum cardiomyopathy (PPCM) is defined as by the European Society of Cardiology as follows: “idiopathic cardiomyopathy presenting with heart failure (HF) secondary to left ventricular (LV) systolic dysfunction toward the end of pregnancy or in the months following delivery, where no other cause of HF is found.” It is a diagnosis of exclusion. The LV may not be dilated but the left ventricular ejection fraction (LVEF) is nearly always reduced below 45%” [1]. PPCM occurs in 0.03% to 0.3% of pregnant women [2-4] with a mortality rate ranging from 4% to 15% [5-8]. Recovery of LVEF (>50%) occurs in approximately 50% of patients within 6 months, but the risk of recurrence of HF in subsequent pregnancies is high in the group where the LVEF has not normalized before the subsequent pregnancy [9]; in the subsequent pregnancies of 16 women with persistent left ventricular dysfunction (LVEF<50%), the mean LVEF (±SD) decreased from 36 ± 9% to 32 ± 11%, symptoms of heart failure occurred in 44% (7/16), and 19% (3/16) died [9]. In counselling, advice against a subsequent pregnancy is recommended in women with LVEF of ≤25% at diagnosis of PPCM or where the LVEF has not normalized [1].
The presenting symptoms in PPCM patients include dyspnea, orthopnea, tachycardia, and peripheral edema [1,7]. The common presenting symptoms, such as dyspnea, orthopnea, and peripheral edema, suggest excessive water retention in patients with PPCM, and greater gestational weight gain (GWG) preceded PPCM exclusively in our previous three PPCM cases encountered over the past decade [10-12]. One of these women became pregnant again 2.5 years later. This case was followed up carefully with respect to cardiac function and GWG, and suggested that limiting GWG was effective in the avoidance of PPCM recurrence. This case is presented with the consent of the patient and approval of the institutional review board of Hokkaido University Hospital. All work was conducted in accordance with the Declaration of Helsinki.
Case Presentation
A 36-year-old Japanese woman became pregnant for the third time 30 months after delivery of the 1st child. Her height was 1.48 m. Clinical details of her first pregnancy at age 33 years complicated with PPCM were described previously [10]. Briefly, in her first pregnancy, GWG was 30 kg (from 38.5 kg at gestational week [GW] 12 to 69.1 kg on the day of delivery at GW 34), and she gave birth to a healthy premature boy weighing 2576 by emergency caesarean section. However, she had suffered from sustained decreased LVEF (<55%); LVEF was 27% on the day of delivery, and 35%, 30%, and 22% on 1.0, 2.6, and 6.0 weeks postpartum, respectively (Figure 1), and 39%, 42%, 52%, and 56% on 9, 12, 18, and 28 months postpartum (2 months prior to the establishment of the current pregnancy). Her second pregnancy at age 35 years ended in miscarriage at GW 7. The current pregnancy (her third pregnancy), carefully monitored with respect to cardiac function with echocardiography and GWG, was uneventful with neither the development of PPCM, hypertension, nor proteinuria. Her pre-pregnancy body weight was 37 kg and GWG was 14.7 kg (51.7 kg on the day of delivery at GW 37), and plasma B-type natriuretic peptide (BNP) level and LVEF remained normal or nearly normal throughout pregnancy (Figure 1). She left hospital on day 10 postpartum after giving birth to a healthy female infant weighing 2325 by repeat caesarean section.
Discussion
The LVEF was 62% in the early stage of the current pregnancy in this patient and PPCM recurrence risk was estimated to be more than 20% based on a previous report [9]; in the subsequent pregnancies of 28 women with normalized LVEF (>50%) after PPCM, more than 20% decrease in LVEF occurred in 21% (6/28) of women and symptoms of heart failure occurred in 21% (6/28) of women [9].
GWG was 14.7 kg in the current pregnancy, while approximately 30 kg in her previous pregnancy complicated with PPCM. Although not verified, we speculated that the much lower GWG in the current pregnancy may have somewhat contributed to the avoidance of LVEF worsening in the current pregnancy based on our experience in three previous PPCM cases, including two other than the present case; both were twin pregnancies and exhibited greater GWG (25.5 kg in one with height of 1.60 m, pre-pregnancy weight of 53 kg, and delivery at GW 32 [11] and 18.3 kg with the greatest weight gain of 6.0 kg during the last four weeks of gestation and the greatest weight loss of 19.2 kg during one month postpartum among 90 women with twin deliveries at GW≥32 in the other with height of 1.56 m, pre-pregnancy weight of 51 kg, and delivery at GW 34 [12]). GWG may vary according to ethnicity. However, among 128838 and 5573 Japanese women with singleton and twin pregnancies, respectively, the numbers of women with GWG>20 kg, >25 kg and >30 kg were 1.1%, 0.20% and 0.06% for women with singleton pregnancies and 3.2%, 0.43%, and 0.09% for twin pregnancies, respectively [13]. Thus, three PPCM cases, including one in a previous pregnancy of the present case, were associated with greater GWG.
The circulating blood volume theoretically increases with increasing GWG. Average GWG reaches a pre-pregnancy body weight of approximately 20% [13], while the circulating blood volume physiologically increases in pregnancy by approximately 40% [14] and this natural volume overload leads to physiological cardiac hypertrophy [15]. However, in a mouse model, pathological hypertrophy (remodeling showing relative lack of capillary density with contractile dysfunction) can occur in the presence of sustained overload [16]. Cardiac biomarkers, such as NT-proBNP and high-sensitive troponin I (TnI), increase significantly during the late stage of pregnancy even in uncomplicated pregnancies, especially in women with well-known risk factors for PPCM, such as preeclampsia and multifetal pregnancy [17]. Both pre-eclampsia and multifetal pregnancy are associated with greater GWG [13,18]. Patients diagnosed with PPCM exhibit high levels of both NT-proBNP and TnI when tested [19]. Thus, greater GWG may be associated with risk of PPCM via greater circulating blood volume expansion.
Experience based on a single case may not deduce any conclusion; however, previous report indicates: 1) PPCM is highly likely to recur, and 2) our data showed that excessive weight gain during pregnancy was a risk factor of PPCM. In counseling, women with a prior history of PPCM associated with greater GWG should be advised to avoid excessive GWG in subsequent pregnancies, although evidence supporting this recommendation is currently lacking.
References
- Sliwa K, HilfikerÃÆâÃâââ¬ÃâÃÂKleiner D, Petrie MC, Mebazaa A, Pieske B, et al. (2010) Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 12: 767-778.
- Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, et al. (2000) Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA 283 1183-1188.
- Desai D, Moodley J, Naidoo D (1995) Peripartum cardiomyopathy: experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature. Tropical Doctor 25: 118-123.
- Fett JD, Carraway RD, Dowell DL, King ME, Pierre R (2002) Peripartum cardiomyopathy in the Hospital Albert Schweitzer District of Haiti. Am J Obstet Gynecol 186: 1005-1010.
- Elkayam U, Akhter MW, Singh H, Khan S, Bitar F (2005) Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation 111: 2050-2055.
- Kamiya CA, Kitakaze M, Ishibashi-Ueda H, Nakatani S, Murohara T, et al. (2011) Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders-results from the Japanese nationwide survey of peripartum cardiomyopathy. Circ J 75: 1975-1981.
- Carlin AJ, Alfirevic Z, Gyte GM (2010) Interventions for treating peripartum cardiomyopathy to improve outcomes for women and babies. The Cochrane Library.
- Sliwa K, Forster O, Libhaber E, Fett JD, Sundstrom JB, et al. (2006) Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J 27: 441-446.
- Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, et al. (2001) Maternal and fetal outcomes of subsequent pregnancies inwomenwith peripartum cardiomyopathy. N Engl J Med 344: 1567-1571.
- Endo D, Morikawa M, Sakakibara M, Sugita T, Yamada T, et al. (2014) Extraordinary weight gain: initial finding in a patient with peripartum cardiomyopathy. Case Rep Perinat Med 3: 45-47.
- Umazume T, Yamada T, Yamada S, Minakami H (2014) Peripartum cardiomyopathy in a woman with preeclampsia with twin 10. pregnancy. BMJ Case Rep.
- Matsumiya H, Saito N, Minakami H, Kataoka S (2015) Gestational Weight Gain and Peripartum Cardiomyopathy in a Twin Pregnancy. Case Rep Obstet Gynecol.
- Morikawa M, Yamada T, Akaishi R, Yamada T, Nishida R, et al. (2014) Gestational weight gain according to number of fetuses in Japanese women. J Perinat Med 42: 523-528.
- Pritchard JA (1965) Changes in blood volume during pregnancy. Anesthesiology 26: 393-399.
- Simmons LA, Gillin AG, Jeremy RW (2002) Structural and functional changes in left ventricle during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol 283: 1627-1633.
- Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, et al. (2005) Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108-2118.
- Umazume T, Yamada T, Ishikawa S, Yamada T, Koyama T, et al. (2015) Prospective study on changes in blood variables in pregnant women at higher risk of peripartum cardiomyopathy ESC Heart Failure 2: 208-215.
- Morikawa M, Yamada T, Yamada T, Sato S, Cho K, et al. (2013) Effect of nulliparity, maternal age, and pre-pregnancy body mass index on the development of gestational hypertension and preeclampsia. Hypertens Res Preg 2: 1-6.
- Huang GY, Zhang LY, Long-Le MA, Le-Xin W (2012) Clinical characteristics and risk factors for peripartum cardiomyopathy. Afr Health Sci 12: 26-31.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences