Factors associated with Synchronous Endometrial and Ovarian Cancer, Review of a Case
Begoña Díaz de la Noval
DOI10.21767/2471-9803.100029
Begoña Díaz de la Noval*
Gynecology-Oncology Unit, Gynecology and Obstetrics Department, University Hospital La Paz, Madrid, Spain
- *Corresponding Author:
- Begoña Díaz de la Noval
Antonio López Aguado St
1, 4th E, 28029, Madrid, Spain
Tel: +34616306566
E-mail: begodelanoval@gmail.com
Received date: June 20, 2016; Accepted date: July 08, 2016; Published date: July 16, 2016
Citation: de la Noval BD. Factors associated with Synchronous Endometrial and Ovarian Cancer, Review of a Case. Crit Care Obst&Gyne. 2016, 2:4. doi: 10.2176 7/2471-9803.100029
Copyright: © 2016 de la Noval BD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Synchronous endometrial and ovarian cancer (SEOC) account from 50% to 70% of all synchronous female genital tract malignancies. The incidence of SEOC is among 5% of women with endometrial cancer (EC) and 10% with ovarian cancer (OC). SEOC are more frequent in younger, obese, premenopausal and nulliparous women. It has been proved that SEOC has a better prognosis than a singleorgan disease with metastasis.
Aim: Our aim is to describe clinicopathologic characteristics and factors associated with SEOC, by reviewing the recent literature published and describing a very representative case.
Keywords
Ovarian cancer, Malignancies, Endometrial cancer, Nulliparous women, Metastasis
Case Report
A 54-year-old perimenopausal woman complained of abnormal uterine bleedings and lower abdominal pain for 6 months. Personal antecedents were smoking, hypertension, chronic obstruction pulmonary disease, obstructive sleep apnea, obesity (BMI 42.7 kg/m2) and nulliparity [1].
The first exploration showed high tumor markers (Ca125 890 UI/ml, Ca19.9 960 UI/ml) and an enlarged uterus with thickened endometrium, but the gynecological examination was unreliable.
A diagnostic hysteroscopy showed an abnormal growth on the entire endometrial cavity with atypical vascularization. Biopsy was consistent with low-grade endometrial adenocarcinoma.
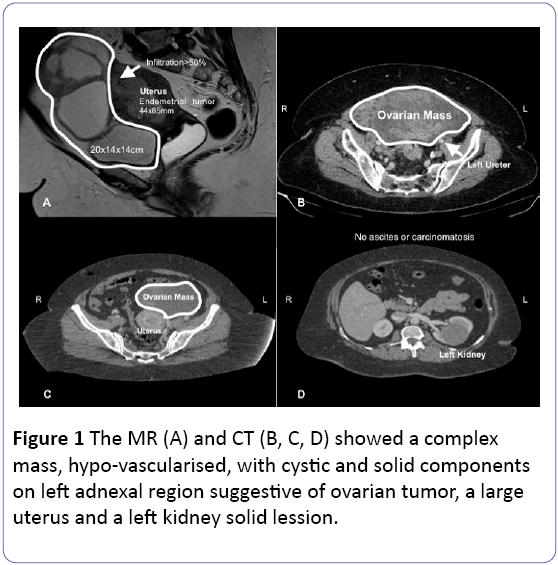
The pelvic magnetic resonance (MR) showed a large uterus, occupied by an endometrial carcinoma 4 mm close to the uterine serosa. There was an invasion to the cervix stromal, a left 20 cm heterogeneous and a hypovascular multiloculated adnexal mass adhered to mesosigma. No ascites or carcinomatosis were found.
A computerized tomography (CT) was performed finding a 6 cm solid left renal tumor suspicious of malignancy, and unspecific pelvic and paraaortic lymph nodes (Figure 1). Histology and radiology raised suspicions of an advanced endometrial cancer and synchronous renal tumor. After reviewing the case, we decided to perform a complete endometrial cancer staging procedure by laparoscopy, with intraoperative analysis of the adnexal mass and left nephrectomy.
In December 2015, the patient underwent surgery. The intraoperative analysis showed an endometrioid endometrial carcinoma invading less than 50% of the myometrium, and a carcinoma whose primary origin could not be confirmed as ovarian.
A laparoscopy was performed, finding a 20 cm left adnexal mass with a large uterus, but no carcinomatosis or ascites. Breathing difficulties forced us to change to laparotomy. The complete staging surgery consisted in: total abdominal hysterectomy, bilateral pelvic, pelviperitonectomy, bilateral salpingo-oophorectomy and paraaortic lymphadenectomy to the level of the left renal vein, total sub-colonic omentectomy, appendectomy, left nephrectomy and splenectomy. Postoperative recovery was complex, with multifactorial shock, pneumonia, and sepsis.
Final histopathology results revealed a synchronous endometrioid endometrial and an ovarian cancer associated to endometriosis. A chromophobe renal carcinoma was also found.
The 4 cm ovarian endometrioid adenocarcinoma presented lymphovascular space invasion, and a stage IIIA1 in the FIGO scale, because of paraaortic lymph node metastasis (a total of 2 in 40 paraaortic and none of 38 pelvic nodes were metastatic from ovarian carcinoma).
The 85 mm endometrioid endometrial adenocarcinoma was a remarkably differentiated FIGO stage IA, classified by the European Society of Medical Oncology (ESMO) as a low risk endometrial cancer.
We decided to complete the treatment with adjuvant standard chemotherapy (Carboplatin-paclitaxel six courses) followed by vaginal brachytherapy (21 Gy) that was well tolerated. The patient is currently free of disease.
Discussion
Synchronous cancers account for 0.7-1.8% of all gynecologic malignancies. Among them, synchronous ovarian and endometrial cancers are the most frequent (40-53%) [2]. Abnormal uterine bleeding is the most often reported symptom associated to EC (36%), which can explain why synchronous OC is also diagnosed at an early stage [3].
The mean age of SEOC patients is 53 years old [3]. Nearly 1 in 10 women under 50 years old that are diagnosed with EC, will have a synchronous OC [4]. Comparing the histologic subtypes, the endometrioid tends to appear in younger patients. In Sozen et al. article, 48% of woman with SEOC were premenopausal [3]. In Oranratanaphan’s et al. study, patients with synchronous cancers were younger, diagnosed at earlier stages, more frequent nulliparous and with better prognosis than the patients in the metastatic group [5].
Risk factors associated with synchronous ovarian and endometrial cancer are hyperestrogenic conditions such as: obesity, perimenopause, chronic anovulation, polycystic ovarian syndrome, estrogen-producing ovarian tumors and unopposed estrogen replacement therapy [1,4]. This suggests that a hormonal “estrogenic effect” may account for the development of these simultaneous cancers [1]. However, most studies have involved a small number of patients, therefore these predictors for tumor development and recurrence have not yet been clearly established [1].
SEOC in patients appears with different clinical characteristics compared to patients with isolated EC/OC, and it is usually classified as a single-organ advanced disease. In patients with abnormal uterine bleeding, the ovaries should be assessed by imaging test; and in patients presenting with an adnexal mass, assessment of the endometrium is essential [6].
The detection of an endometrial and ovarian synchronic tumor is very important, to perform an accurate complete staging surgery, that could avoid reintervention and adjuvant treatment.
In this particular case, we had the suspicion of an ovarian cancer, therefore a pelvic and paraaortic lymphadenectomy was performed, in order to give the patient a proper adjuvant therapy. In addition to this, our patient had multiple risk factors, so she was not eligible for a new surgery after histologic confirmation. In case of suspicion or doubt, an intraoperative study of the tumor specimen is recommended. On the other hand, in young patients those want to preserve fertility, an appropriate treatment plan before surgery will be beneficial [6]. Lymphadenectomy should be performed in every patient with SEOC [7].
As determined in our case, in SEOC, ovarian tumor is usually unilateral, lymphovascular space invasion is related to the ovarian carcinoma and lower Ca125 level at diagnosis [7]. Consistent pathologic criteria are crucial to distinguishing between synchronous and metastatic disease [4] Scully et al. described a list of clinicopathologic postoperatively features used to differentiate endometrial disease with metastasis to the ovary, from ovarian disease with metastasis to the endometrium; updated by Ulbright and Roth (Table 1) [3,8]. Histopathological diagnosis of SEOC still lacks a clear definition; immunohistochemistry and molecular analysis are very useful to a more accurate diagnosis [6,9].
| No. | Primary Endomentrial Cancer with ovarian metastases (ECOM) | Primary Ovarian Cancer with endomentrial metastatses (OCEM) | Independent Primary Endomentrial and Ovarian tumors (SEOC) |
|---|---|---|---|
| 1 | Histologic similarity of the tumors | Histologic similarity of the tumors | Histologic dis-similarity of the tumors |
| 2 | Large endomentrial and small ovarian tumors | Large ovarian and small endomentrial tumors | |
| 3 | Atypical endomentrial hyperlasia additionally present | No atypical endomentrial hyperlasia | Atypical endomentrial hyperlasia |
| 4 | Deep myometrial invasion. Direct extension into the adnexa. Vascular space invasion in myometrium, surface implants or combination in ovary. | Location in ovarian parenchyma. Direct extension from ovary into outer wall uterus. Ovarian tumor located in parenchyma. | No or only superficial myometrial invasion of endometrial tumor. No vascular space invasion, surface implants or predominant hilsr location in parenchyma. Ovarian tumor located in parenchyma. |
| 5 | Spread elsewhere in typical pattern of endomentrial carcinoma. | Spread elsewhere in typical pattern of ovarian carcinoma. | Abesnce of other evidence of spread of ovarian tumor. |
| 6 | Ovarian tumor bilateral and/ or multinodular | Ovarian tumor unilateral (80-90% of cases) in a single mass. | Ovarian tumor unilateral (80-90% of cases). |
| 7 | Ovarian endometriosis absent. | Ovarian endometriosis absent. | Ovarian endometriosis absent. |
| 8 | Aneuploidy with similar DNA indices or diploidy of both tumors*. | Aneuploidy with similar DNA indices or diploidy of both tumors*. | Different ploidy of DNA indices, if aneuploidy, of the tumors*. |
| 9 | Similar molecular genetic karyotypic abnormalities in both tumors. | Similar molecular genetic karyotypic abnormalities in both tumors. | Dissimilar molecular genetic or karyotypic abnormalities in both tumors. |
| ECOM: Endometrial Cancer and Ovarian Metastases; OCEM: Ovarian Cancer and Endometrial Metastases; SEOC: Synchronous Endometrial and Ovarian Cancer. * The possiblity of tumor heterogeneity must be taken into account in the evaluation of ploidy findings. | |||
Table 1: The clinicopathological differential diagnostic criteria of SEOC described by Scully, et al.
The most common histological type of both endometrial and ovarian cancers is endometrioid carcinoma [2]. Endometrioid cancer is an uncommon histological type of ovarian cancer and it is thought to be developed under the same conditions as endometrial cancer. The exact etiology of synchronous primary cancers is unknown, [5] but endometriosis and a hyperestrogenic environment may be involved [3,5]. However, further studies should be developed in order to confirm that hypothesis.
SEOC are usually diagnosed at an earlier stage (48% FIGO stage I for both EC and OC) and lower histological grade [2,6]. In Oranratanaphan et al. study most patients (85.7%) presented an early stage and only 14.3% in advanced stage of OC [5]. Our patient had been complaining of vaginal and abdominal bleeding for a period of 6 months, probably that delay in the diagnosis was the key for an advanced ovarian cancer.
Patients with endometrioid endometrial histology and endometrioid ovarian histology are usually thought to have a better prognosis, compared to the other histologic types or metastatic ovarian and endometrial cancers. Nearly one-third of the patients had a recurrence. [3] In the article published by Heitz et al., they conclude that after the calculation of the prognosis for each tumor stage, the overall survival (OS) or progression-free survival (PFS) does not change [9].
The prognosis of patients with SEOC with different tumor FIGO stages, like our case, seems to be mainly dependent on the prognosis of the advanced cancer [9]. General prognostic factors related were: young age, premenopausal status, no obesity, low FIGO stage, low-grade and low-risk histology, low level of cancer antigen Ca125 at diagnosis, complete staging or debulking surgery and good performance status [9]. For Bese et al., other prognostic factors are: performing lymphadenectomy, an absence of omental metastasis, and no residual disease [7].
High level of Ca125 at diagnoses was an independent risk factor for recurrence [3]. In a multicenter study by Song et al., pre-treatment serum Ca125 and tumor stage of the ovary were found to have prognostic significance on PFS and OS [1] Sozen H et al., concluded that synchronous endometrioid type, in primary EC and OC, had different clinical histopathological characteristics and favorable prognosis, compared to the other histologic types of these tumors [3]. Oranratanaphan et al., estimated that 5-year PFS was 64% and OS was 92.8%, compared to 41% and 48.5% in EC with ovarian metastasis [5].
Treatment recommendations should take into account the risk of relapse of the respective tumor compounds. Therapy usually depends on the stage and grade of ovarian cancer, because this neoplasm is characterized by a worse prognosis and greater risk of recurrence [9]. The main adjuvant treatment is chemotherapy, followed by radiotherapy, but is more controversial if indicated [2]. In our case only chemotherapy was used, because radiotherapy requirements were not meet.
Although we only report about one single case with a short follow-up and in retrospect, it is representative for the subject of our analysis. It would be appropriate to conduct a comprehensive analysis of cases, or a multicenter study with longer follow-up. In order to determinate the prognosis of adjuvant treatment by stage, a comparison between SEOC, primary EC with ovarian metastases (ECOM) and primary OC with endometrial metastases (OCEM) should be conducted.
We conclude that in the case of a suspected synchronous tumor, Scully et al. clinicopathologic criteria [10] and intraoperative assessment are recommended. More aggressive therapeutic approaches should be considered, for patients with SEOC, in order to perform a complete surgery and adjuvant therapy. More prospective studies would be required.
References
- Song T, Seong SJ, Bae DS, Kim JH, Suh DH, et al. (2014) Prognostic factors in women with synchronous endometrial and ovarian cancers. Int J Gynecol Cancer 24: 520-527.
- DÃÆââ¬Å¾Ãâââ¢bska-Szmich S, Czernek U, Krakowska M, FrÃÆââ¬Å¾Ãâââ¬Â¦ckowiak M, ZiÃÆââ¬Å¾Ãâââ¢ba A, et al. (2014) Synchronous primary ovarian and endometrial cancers: a series of cases and a review of literature. Prz Menopauzalny 13: 64-69.
- Sozen H, Vatansever D, Iyibozkurt AC, Topuz S, Ozsurmeli M, et al. (2015) Clinicopathologic and survival analyses of synchronous primary endometrial and epithelial ovarian cancers. J Obstet Gynaecol Res 41: 1813-1819.
- AlHilli MM, Dowdy SC, Weaver AL, Sauver JL, Keeney GL, et al. (2012) Incidence and Factors Associated with Synchronous Ovarian and Endometrial Cancer: A Population-Based Case-Control Study. Gynecol Oncol 125: 109-113.
- Oranratanaphan S, Manchana T, Sirisabya N (2008) Clinicopathologic variables and survival comparison of patients with synchronous endometrial and ovarian cancers versus primary endometrial cancer with ovarian metastasis. Asian Pac J Cancer Prev 9: 403-407.
- Broeders FM, van der Wurff AA, Pijnenborg JM, Vos MC (2012) Preoperative identification of synchronous ovarian and endometrial cancers: the importance of appropriate workup. Int J Gynecol Cancer 22: 1325-1331.
- Bese T, Sal V, Kahramanoglu I, Tokgozoglu N, Demirkiran F, et al. (2016) Synchronous Primary Cancers of the Endometrium and Ovary With the Same Histopathologic Type Versus Endometrial Cancer With Ovarian Metastasis: A Single Institution Review of 72 Cases. Int J Gynecol Cancer 26: 394-406.
- Ulbright TM, Roth LM (1985) Metastatic and independent cancers of the endometrium and ovary: a clinicopathologic study of 34 cases. Human pathology 16: 28-34.
- Heitz F, Amant F, Fotopoulou C, Battista MJ, Wimberger P, et al. (2014) Synchronous ovarian and endometrial cancer, an international multicenter case-control study. Int J Gynecol Cancer 24: 54-60.
- Scully RE (1979) Tumors of the ovary and maldeveloped gonads. Washington, D.C: Armed Forces Institute of Pathology.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences